August 28, 2018
Written by: Carolyn Keating
Pregnancy is one of the most drastic changes a woman can undergo. Not only does she experience a major physical transformation, but it turns out that her brain (permanently!) changes as well.
Pregnancy affects virtually every system in the body. Organs shift around to accommodate the growing baby (resulting in respiratory and gastrointestinal discomfort), breathing rate rises to support the increased oxygen needs of the mother’s and fetus’s tissues, the heartrate and volume of blood increases, kidneys are working overtime to manage the waste from mom and baby, and of course, the endocrine system that produces hormones goes crazy. But hormones such as estrogen, progesterone, and prolactin don’t just help adjust the body for pregnancy and birth. They also affect the function of the brain, and its very structure as well.
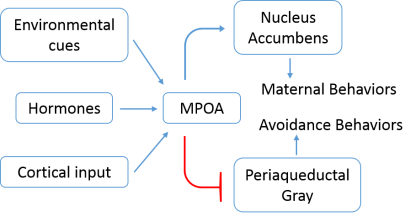
Hormones are an important driver of maternal behavior: actions a mother takes to care for and raise her young. These behaviors are thought to arise from a discrete neural network in the brain (which has mainly been studied in rodents). One important region is the medial preoptic area (MPOA), a tiny center in a region called the hypothalamus. This area is thought to be a key site for the integration of hormone signals, information from the environment, and inputs from other brain regions, and stimulates the onset and continuance of basic maternal behaviors (Figure 1). After integration, the MPOA facilitates both the fulfilling feeling of maternal care, while discouraging avoidance behaviors. To support maternal behavior, the MPOA sends information to the ventral tegmental area, which in turns projects to the nucleus accumbens, a brain region involved with mediating motivation (so basically, reinforcing maternal behaviors by making them rewarding). At the same time, the other neurons in the MPOA (along with other regions) inhibits an area called the periaqueductal gray to reduce avoidance behavior and increase the likelihood that the mother will find her new baby attractive1. Importantly, estrogen and progesterone have been shown to enlarge the cell bodies of neurons in the MPOA, as well as increase the number of dendritic branches—the parts of the cell that receive inputs from other neurons2. Furthermore, during pregnancy the MPOA increases the number of hormone receptors on its neurons, making the mother more sensitive to estrogen and further stimulating maternal care1.
But the MPOA and associated maternal neural network aren’t the only brain regions affected by pregnancy. Areas controlling learning and memory also change. The hippocampus, an area involved with spatial learning and memory, is sensitive to reproductive hormones. Elevated estrogen levels lead to increases in dendritic spines and the number of excitatory synapses in the hippocampus3,4, and rat mothers have enhanced spatial memory in foraging tests, when animals must search for and remember the location of food for their pups5. These changes are long-lasting: improved spatial memory was seen for the duration of mother rats’ lives after giving birth6.
But what about humans? Although people don’t exactly display foraging behavior the way rodents do, they do have important hormone-mediated maternal behaviors that are more related to social and emotional processing. For instance, researchers have found that in late pregnancy (when hormones are at their highest levels), mothers are better at processing emotional faces7. Another study found that oxytocin levels during and right after pregnancy correlated with displays of maternal behavior such as gazing at the infant’s face vocalizations, and affectionate touch8.
Are there structural changes that accompany these maternal behaviors in humans as there are in rodents? Research published last year shows that the answer is a resounding “yes.” In a first of its kind study9, scientists used MRI to examine the brains of mothers before and 2 years after their first pregnancy. They found considerable changes in gray matter—the part of the brain where the neuronal cell bodies reside—after pregnancy. More specifically, researchers saw decreases in gray matter volume in regions of the brain involved with social cognition. In fact, in the same regions are involved with theory-of mind, which is the ability to recognize and attribute mental states like thoughts, perceptions, desires, intentions, and feelings, to oneself and others and to understand how these mental states might affect behavior. And these changes were long-lasting—they were still apparent two years after the women had given birth!
Although the study found no changes in cognitive performance before and after pregnancy (and although they did not perform any cognitive assessments during pregnancy, pregnancy brain might not be a real thing anyways), they did show that the more gray matter reduction a woman had, the greater her attachment to her new baby, meaning absence of hostility and pleasure in interactions with the newborn.
So is losing gray matter a good thing? When most people hear about decreased volume of brain tissue, they assume that’s a bad sign. But loss of gray matter does not necessarily mean loss of neurons. It could also reflect difference in the numbers of supporting glial cells, changes in vasculature or blood volume, increases in myelination, or changes in the number of synapses. And it is this last point—changes in numbers of synapses—that the authors speculate could make this reduction in gray matter volume beneficial. Synapses are “pruned”—that is, unnecessary connections between cells are removed—during another period in everyone’s lives: puberty (which also happens to be a time of major hormone changes). In teenagers, synaptic pruning is believed to be a crucial process for fine-tuning the brain’s circuitry that is vital for proper development. Although the MRI research was not designed to study mechanisms of gray matter loss, the authors suggest that much like in puberty, the brains of mothers also undergo maturation and specialization. The theory-of-mind brain network is considered to be essential for maternal behaviors in humans—enhancing a parent’s abilities to recognize her child’s needs or identify potential threats—so it makes sense that this system would be refined in mothers.
It’s clear that not only do women’s bodies undergo profound changes during pregnancy, but their brains do as well. But these changes seem to be for the better. Life changes a lot when you have a child—your activities, priorities, and needs all shift—and it appears that our brains are programmed to make this time of intense adjustment just a little bit easier.
References
- Bridges, R. S. Neuroendocrine regulation of maternal behavior. Front. Neuroendocrinol. 36, 178–196 (2015).
- Keyser-Marcus, L. et al. Alterations of medial preoptic area neurons following pregnancy and pregnancy-like steroidal treatment in the rat. Brain Res. Bull. 55, 737–745 (2001).
- Woolley, C. S. & McEwen, B. S. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci. 12, 2549–2554 (1992).
- Woolley, C. S., Weiland, N. G., McEwen, B. S. & Schwartzkroin, P. A. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J. Neurosci. 17, 1848–1859 (1997).
- Kinsley, C. H. et al. Motherhood improves learning and memory. Nature 402, 137–138 (1999).
- Love, G. et al. Maternal experience produces long-lasting behavioral modifications in the rat. Behav. Neurosci. 119, 1084–1096 (2005).
- Pearson, R. M., Lightman, S. L. & Evans, J. Emotional sensitivity for motherhood: late pregnancy is associated with enhanced accuracy to encode emotional faces. Horm. Behav. 56, 557–563 (2009).
- Feldman, R., Weller, A., Zagoory-Sharon, O. & Levine, A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol. Sci. 18, 965–970 (2007).
- Hoekzema, E. et al. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 20, 287–296 (2017).
