June 18th, 2024
Written by: Jafar Bhatti
In our daily lives, we occasionally find ourselves in new and exciting environments, like when we are on vacation exploring a new city. However, more often than not, we spend time in environments that are familiar, like when we travel to work using the same daily commute. For a long time, neuroscientists thought that brain activity is unchanging in these familiar and routine environments. However, new research is challenging this assumption by showing that brain activity can surprisingly “drift” despite seemingly unchanging environments. In this post, we will dive into this emerging dilemma that the field of neuroscience is beginning to address.
Our brain as a representation of the world
Often, neuroscientists talk about “representations” in brain activity that correspond to something in the world around us. What does this mean exactly? A “neuronal representation” refers to the activity of single or multiple brain cells, better known as neurons, whose activity appears to reflect some real-world event. This real-world event can be anything – from the feeling of pain after a toe stub to the memories we recall from our childhood. In neuroscience, the set of neurons that collectively form a neuronal representation is given a special name – a neuronal ensemble.
When we are presented with a new or changing environment, like the example given earlier of navigating a new city, neuroscientists have found evidence that the neuronal ensembles that represent this new environment changes as you continue to learn. Much of this work comes from rodents (see Linday’s wonderful post on the immense benefits of rodent research). Indeed, many studies have shown that rodents introduced to a new space develop new “spatial maps” that help the rodent navigate their way around[1]. These maps are formed by a specific neuronal ensemble, and as expected, the neurons that make up the ensemble change if the rodent is again placed in a novel environment[2].
What about a familiar environment, like in the example given earlier of commuting to work? For a long time, most neuroscientists would say that the neuronal ensemble that tracks your commute to work is likely to be the same, since you have already learned your commute and the commute to work is the same every day. However, this hypothesis could not be tested directly until recently because neuroscientists didn’t have technology that allowed them to record from the same neurons over long periods of time. The advent of advanced neuronal recording technologies has allowed researchers to collect single-neuron recordings over multiple days or even weeks. Recent work using this technology in mice has shown that the neuronal ensembles that track a particular space can change dramatically over the course of days and weeks, even when traveling the same exact space[3]. (Figure 1). In other words, the researchers found that this ensemble demonstrates representational drift – an ongoing change in neuronal representation that occurs without obvious changes in behavior or the environment[4].
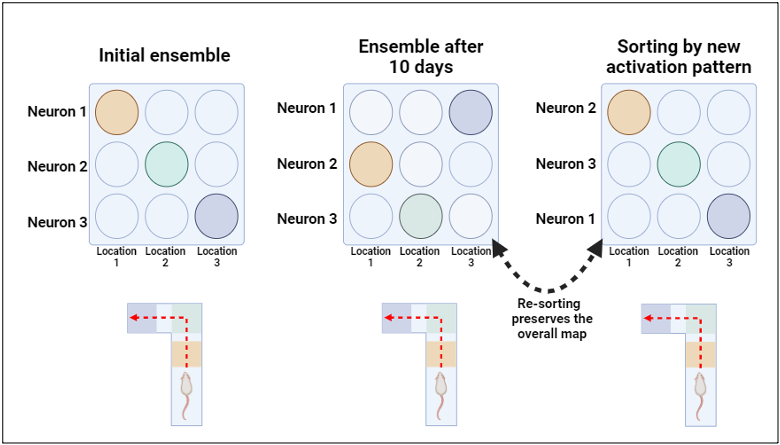
The broad scope of representational drift
The issue of representational drift appears to be quite large in scope. Firstly, while the above study focused on representational drift located in one particular brain region, other studies have found representational drift in other brain regions involved in navigation[5],[6], highlighting that this phenomenon is not limited to just one particular brain area. Secondly, the issue of representational drift appears to go beyond just navigation as one group found that representational drift can occur for memories of learned experiences in rodents[7]. Thirdly, and perhaps most importantly, representational drift has been found in species other than mice. Researchers have found representational drift can take place over the course of minutes and hours in rhesus macaque monkeys during tasks involving directing arm movements[8],[9]. This finding is perhaps most surprising as the rhesus macaque brain closely resembles those of humans and therefore provides strong evidence that representational drift may be a natural phenomenon that occurs in our brains as well.
Some neuroscientists argue that the broadness of neuronal drift is interesting, but not all too surprising, since these particular examples of neuronal representations are very complex to begin with (i.e., spatial maps, memory, motor planning). Perhaps it’s the case that neuronal drift only occurs for complex representations, like in the earlier example of navigation. If this hypothesis were true, we would expect that neuronal ensembles for more simple sensory information would not undergo representational drift. Contrary to this hypothesis, recent work by multiple research groups have found evidence for representational drift in the brain areas responsible for very simple neuronal representations [10], [11].
Conclusion
Given that representational drift takes place over the course of hours, days, and weeks and seems to occur at all levels and across brain areas, researchers are now beginning to ask – why? Why has evolution made the brain such that representations that we expect to be stable and long-lasting are actually constantly changing? The short answer is that no one knows. Although some researchers have begun to speculate what purpose representational drift may serve, little is currently known for sure.
There is certainly a lot of work to be done in understanding the nature of representational drift. For example, how is representational drift different between species? How about in old age? And does neuronal drift happen more in certain types of neurons than others? These questions and other will be important as the field of neuroscience moves towards implementing brain-computer interfaces, which often rely on stable readout of neuronal activity. As neuroscience technology continues to improve, I expect that we will soon have better insight into what purpose representational drift serves and how it fits into our larger understanding of the inner workings of the brain. That is, if you catch my drift.
References
[1] O’Keefe, J. & Dostrovsky, J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171-175.
[2] Fenton AA. Remapping revisited: how the hippocampus represents different spaces. Nat Rev Neurosci. 2024 Jun;25(6):428-448. doi: 10.1038/s41583-024-00817-x. Epub 2024 May 7. PMID: 38714834.
[3] Driscoll LN, Pettit NL, Minderer M, Chettih SN, Harvey CD. Dynamic Reorganization of Neuronal Activity Patterns in Parietal Cortex. Cell. 2017 Aug 24;170(5):986-999.e16. doi: 10.1016/j.cell.2017.07.021. Epub 2017 Aug 17. PMID: 28823559; PMCID: PMC5718200.
[4] Rule ME, O’Leary T, Harvey CD. Causes and consequences of representational drift. Curr Opin Neurobiol. 2019 Oct;58:141-147. doi: 10.1016/j.conb.2019.08.005. Epub 2019 Sep 27. PMID: 31569062; PMCID: PMC7385530.
[5] Singh A, Peyrache A, Humphries MD. Medial Prefrontal Cortex Population Activity Is Plastic Irrespective of Learning. J Neurosci. 2019 May 1;39(18):3470-3483. doi: 10.1523/JNEUROSCI.1370-17.2019. Epub 2019 Feb 27. PMID: 30814311; PMCID: PMC6495133.
[6] Attardo A, Lu J, Kawashima T, Okuno H, Fitzgerald JE, Bito H, Schnitzer MJ. Long-Term Consolidation of Ensemble Neural Plasticity Patterns in Hippocampal Area CA1. Cell Rep. 2018 Oct 16;25(3):640-650.e2. doi: 10.1016/j.celrep.2018.09.064. PMID: 30332644.
[7] Cho HY, Shin W, Lee HS, Lee Y, Kim M, Oh JP, Han J, Jeong Y, Suh B, Kim E, Han JH. Turnover of fear engram cells by repeated experience. Curr Biol. 2021 Dec 20;31(24):5450-5461.e4. doi: 10.1016/j.cub.2021.10.004. Epub 2021 Oct 22. PMID: 34687608.
[8] Carmena JM, Lebedev MA, Henriquez CS, Nicolelis MA. Stable ensemble performance with single-neuron variability during reaching movements in primates. J Neurosci. 2005 Nov 16;25(46):10712-6. doi: 10.1523/JNEUROSCI.2772-05.2005. PMID: 16291944; PMCID: PMC6725856.
[9] Rokni U, Richardson AG, Bizzi E, Seung HS. Motor learning with unstable neural representations. Neuron. 2007 May 24;54(4):653-66. doi: 10.1016/j.neuron.2007.04.030. PMID: 17521576.
[10] Marks TD, Goard MJ. Stimulus-dependent representational drift in primary visual cortex. Nat Commun. 2021 Aug 27;12(1):5169. doi: 10.1038/s41467-021-25436-3. Erratum in: Nat Commun. 2021 Sep 10;12(1):5486. doi: 10.1038/s41467-021-25825-8. PMID: 34453051; PMCID: PMC8397766.
[11] Schoonover CE, Ohashi SN, Axel R, Fink AJP. Representational drift in primary olfactory cortex. Nature. 2021 Jun;594(7864):541-546. doi: 10.1038/s41586-021-03628-7. Epub 2021 Jun 9. PMID: 34108681.
Cover photo by Maab Hassan from pixabay.com
Figure 1. adapted from Driscoll et al., 2017 (Reference 3) and re-created by Jafar Bhatti with BioRender.com

Leave a comment